Sometimes it seems like even the most harmless of falls will crack a smartphone screen. Getting a protective case and a phone with Gorilla Glass will help, but almost nothing will stop a screen from cracking from a hard fall.
To solve this dilemma, Akhan Semiconductor is set to release the world’s first smartphone that uses a “Miraj Diamond Glass” screen.(1) While the name might cause one to initially think of a smartphone-sized diamond being sliced very thin to make a screen, that’s not really possible. Natural diamonds seldom are that big
Instead, lab-created diamonds are formed into a nanocrystalline pattern. This pattern avoids weak spots in the glass and helps resist cracking. While it won’t make the screen indestructible, it should make it ultra-resistant to cracking from drops.
While all this may sound great, there are a few drawbacks. First, Akhan has to figure out a way to keep the screen from being too shiny. Diamonds are known for being sparkly, but this is a detriment when it comes to looking at a phone screen in bright conditions. The reflection could make the screen hard to see. Akhan is working on solving this.
Second, making lab-created diamonds is an expensive process. Because of the cost involved, don’t expect to see diamond glass on lower and mid-end phones. Only the high-end phones will see this innovation, at least initially. After all, with the rapidity of new releases and updates in the cell phone market, it would be hard to justify a super-expensive screen, no matter how durable, on a low or mid-end phone that will likely be replaced in a year or two.
According to Akhan, it is possible that a phone with diamond glass may appear in 2019.(2) If and when one of these phones is released, expect a slick marketing campaign in order to get consumers to part with their hard-earned dollars.
Only time will tell if buyers will opt for a more expensive, more durable screen over simply buying some sort of screen replacement protection package. If Akhan is able to price their screen at least relatively close to the cost of a screen replacement package, they may well have a big hit on their hands.
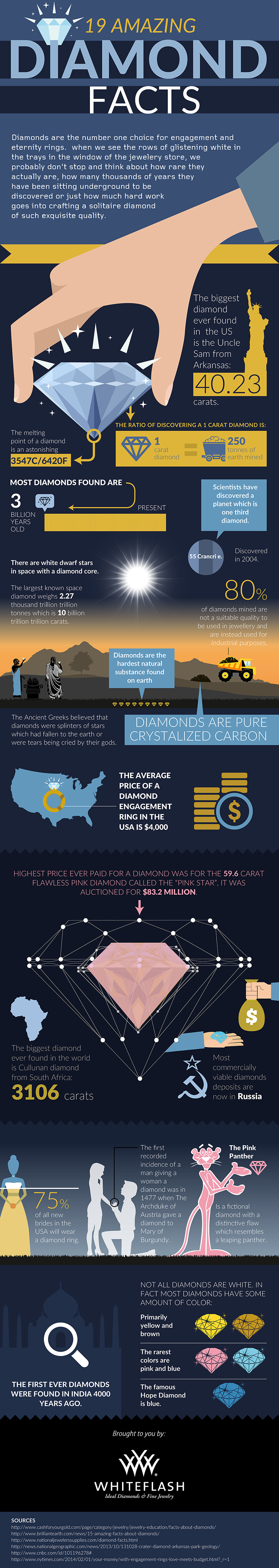

 Geochemist at Columbia University, Yaakov Weiss says, “We can look at diamonds as time capsules, as messengers from a place we have no other way of seeing.” This is an interesting way to conceptualize the expensive precious stones that have been a symbol of affluence as long as time.
Geochemist at Columbia University, Yaakov Weiss says, “We can look at diamonds as time capsules, as messengers from a place we have no other way of seeing.” This is an interesting way to conceptualize the expensive precious stones that have been a symbol of affluence as long as time.